Abstract
The objective of this study was to investigate the relation between habitual milk and dairy consumption and brain morphology as assessed by magnetic resonance imaging (MRI) investigations in 119 young healthy university students. MRI measurements were performed on a Siemens Magnetom Trio Tim (3 T) system while Freesurfer software suite was used for volumetric segmentation. Dietary habits related to milk and dairy consumption were assessed by a structured questionnaire. Total cerebral cortex, total cerebral white matter and total cerebral parenchyma were significantly related with cottage cheese and total protein intake from milk and dairy also when controlled for age and gender in the multivariate model. Our results indicate that dietary habits related with milk and dairy are proportionally associated with volumes of both cerebral cortex and cerebral white matter.
Authors
Gergely Darnai1, Enikő Plózer1, Anna Altbäcker1, Gábor Perlaki1,2,3, Gergely Orsi1,2,3, Szilvia Anett Nagy2, Réka Horváth1, Attila Schwarcz3,4, Norbert Kovács1, József Janszky1,3 and Zsófia Clemens1,5
1Department of Neurology, University of Pécs, Pécs, Hungary
2Pécs Diagnostic Centre, Pécs, Hungary
3MTA-PTE Clinical Neuroscience MR Research Group, Pécs, Hungary
4Department of Neurosurgery
5National Institute of Clinical Neuroscience, Budapest, Hungary
Keywords: milk consumption; cheese; cottage cheese; total milk-diary protein; brain volumetry
Highlights
• Cheese consumption positively correlated with TCC, TWM and TCC.
• Cottage cheese consumption positively correlated with TCC, TWM and TCC.
• Total milk-protein intake positively correlated with TCC, TWM and TCC.
• Cottage cheese consumption’s effect shows gender differences.
Introduction
Structure and function of human brain is thought to be influenced by dietary factors (Gómez-Pinilla, 2008). Several neurological conditions including dementia were suggested to result from carbohydrate overconsumption, lack of essential fatty acids and shortage of vitamins (Gómez-Pinilla, 2008). From an evolutionary perspective, cow’s milk is relatively new in human nutrition (Cordain et al., 2005). In addition, consumption of milk, a cheap source of protein, has increased over the past few decades (Melnik et al., 2012). There are indications that cow’s milk and dairy are implicated in pathological conditions, including type 1 diabetes (Gerstein, 1994), insulin resistance (Melnik et al., 2013) and certain forms of cancer (Melnik et al., 2012). Cow’s milk consumption has consistently been related to accelerated growth in children (Hoppe et al., 2006; Thorsdottir and Thorisdottir, 2011). Growth promoting effect was suggested to result from proteins in cow’s milk such as the insulin like growth factor (IGF-1). We hypothesized that consumption of cow’s milk and dairy may also affect brain structure. To assess this we designed a study to investigate the relation between habitual milk and dairy consumption and brain morphology as assessed by magnetic resonance imaging (MRI) investigations in young healthy university students.
Methods
Subjects
One hundred nineteen (48 males and 71 females) healthy university students between the ages of 18 and 30 were recruited through advertisements placed on notice boards at the University of Pécs, Hungary. Subjects were free of brain disorders, drug or alcohol abuse. Subjects underwent MRI measurements and filled out a set of questionnaire assessing psychological sphere and life style including habitual food intake with a special emphases on milk and dairy products. The study was approved by the local ethical committee of the University of Pécs and all subjects gave written informed consent.
Magnetic resonance imaging examinations
Magnetic resonance imaging (MRI) measurements were performed on a Siemens Magnetom Trio Tim (3 T) system (Siemens AG, Erlangen, Germany), with a 12-channel head coil. For the volumetric analysis a T1 weighted sagittal magnetization-prepared rapid acquisition with gradient echo (MPRAGE) images were used: field of view = 256 × 2562 mm, repetition time= 2530 ms, echo time = 3.37 ms, TI = 1100 ms, slice thickness = 1 mm, slice number = 176, flip angle=7°, bandwidth = 200 Hz/pixel, 256 × 256 matrix.
MRI data post processing
Freesurfer 4.5.0 software suite was used for cortical reconstruction and volumetric segmentation of the images (http://surfer.nmr.mgh.harvard.edu/). Freesurfer software can be used for reliable automated brain segmentation for structures and allows us to assess the volume of the pre-defined brain structures. Freesurfer’s semi-automatic anatomical processing
steps were executed on all subjects’ data including removal of the non-brain tissue, the automated Talairach transformation, volumetric segmentation, intensity normalization, tessellation of borders between the grey matter and white matter surfaces and using intensity gradients to localize the grey matter, white matter, and fluid boundaries. Accuracy of the Talairach transformation, skull strip, white matter and pial surfaces and subcortical segmentation were visually checked in case of every subject after each script. Error correction was applied where it was necessary. The final segmentation is based on a probabilistic atlas and the subject-specific measured values. The following structures were used for statistical analysis: total intracranial volume (ICV), total cerebral white matter (TCWM), the left hemisphere cortical grey matter and right hemisphere cortical grey matter summarized as total cerebral cortex (TCC), total ventricular volume (TVV) which was the sum of the left and right lateral ventricle, left and right inferior lateral ventricle, third and fourth ventricles. Additionally we calculated total cerebral parenchyma (TCP) which was a sum of TCWM and TCC.
Milk-dairy questionnaire
Dietary habits related to milk and dairy consumption were assessed by a structured questionnaire. This questionnaire addressed the following items: full fat milk, reduced fat milk, skimmed milk, soft cheese, semi-hard cheese, hard cheese, cottage cheese, yoghurt, kefir and sour cream. Subjects were asked about how often a particular item was consumed. When answering this subjects had to choose one of the following options: “never”, “less than once a month”, “once a month”, “a few times a month”, “once a week”, “a few times a week”, “once a day”, “a few times a day”. Subjects also had to estimate the weight/volume of the particular items consumed at one occasion.
Milk-dairy estimates
First frequency answers were transformed to weekly frequency estimates such that the eight consecutive categories from “never” to “a few times a day” were transformed to the following numbers: 0, 0.125, 0.25, 0.625, 1, 4, 7 and 14. Then these weekly frequency estimates were multiplied with the reported average weight (or volume) of each item to assess total weekly intake of a particular item. Weekly milk consumption was calculated as a sum of full fat milk, reduced fat milk and skimmed milk while weekly cheese consumption was calculated as a sum of consumption of soft, semi-hard and hard cheese. To create a proxy for total intake of milk and dairy, total milk-dairy protein intake was calculated. Total milk-dairy protein intake was calculated by summing up protein contained by the weekly weight estimates of milk-dairy items. In doing this we calculated with the following protein data: milk (all three types): 3 g/1dl, soft cheese: 1.79 g/10 g, semi-hard cheese: 2.5g/10 g, hard cheese: 2.8 g/10 g, cottage cheese: 1.62 g/10 g, sour crème: 0.28 g/ 10 g, yogurt: 0.3g/10 g, kefir: 0.3g/10 g. The reason for choosing the measure of total protein intake was that milk and dairy products largely differ in their water content thus summing up weight of different dairy types would not be appropriate. A second reason for this was that physiological effects of milk-dairy products are thought to be mediated through the protein found in milk and dairy (Melnik et al., 2012).
Statistical analyses
Data analysis was performed using SPSS statistical software ® version 19.0 (SPSS, Inc., Chicago, IL). First Pearson correlation coefficients were calculated between MRI measurements and milk-dairy parameters. In a second step we controlled for the effect of age and gender in these correlations such that we created separate multiple linear regression models for parameter pairs that were revealed to be significant in the bivariate analysis. The assumptions of multiple linear regressions were satisfied, as judged by testing for linearity, normality assumptions of the residues, outliers, independence of errors, homoscedasticity, and multi-collinearity (Chan, 2004).
Results
Bivariate analyses
Volume of TCC significantly correlated with consumption of cheese (r=0.208, p=0.023), cottage cheese (r=0.197; p=0.032) and total milk-dairy protein (r=0.245, p=0.007).
TCWM volume significantly correlated with consumption of cheese (r=0.243; p=0.008), cottage cheese (r=0.201; p=0.029) and that of milk-dairy protein (r=0.258, p=0.005).
There was a significant positive correlation between TCP and consumption of cheese (r=0.242, p=0.008), cottage cheese (r=0.212, p=0.021) and intake of milk-dairy protein (r=0.269, p=0.003).
ICV and TVV were not significantly correlated with any of the milk-diary parameters.
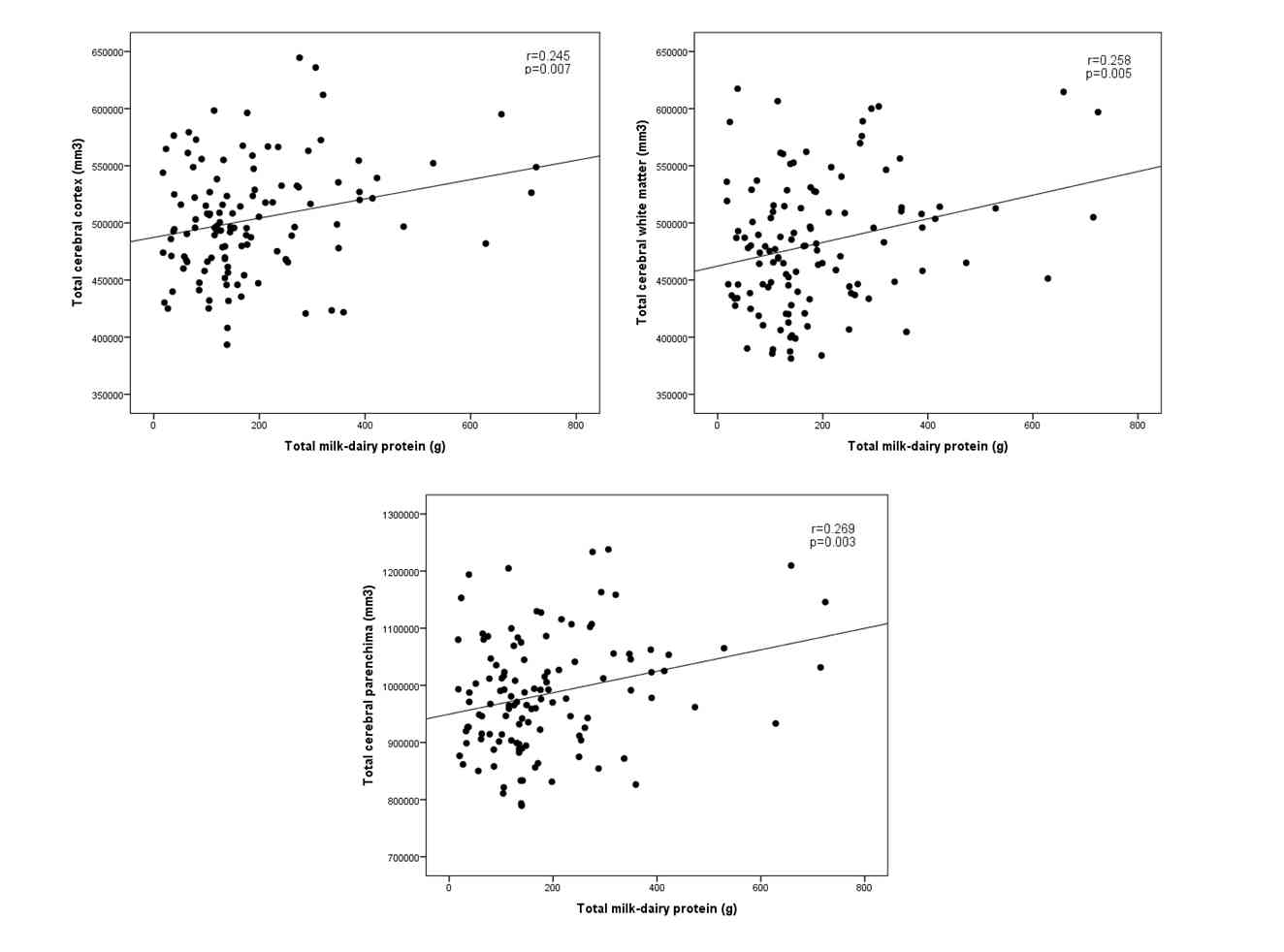
Figure 1
Scatterplot representing the relationship between weekly milk-dairy protein intake and the respective brain volumes. All three correlations remained significant after correction for gender and age.
Multivariate analysis controlling for age and gender
Milk-dairy parameters that proved to be significant in bivariate correlations were entered in multivariate analyses to control for age and gender. In this analysis the significant relationship between TCC and cheese consumption turned to non-significant (beta=0.099, p=0.158) while the relationship between TCC and cottage cheese consumption remained significant (beta=0.214, p=0.001). The relationship between TCC and milk-dairy protein was also significant in the multivariate model (beta=0.151, p=0.029).
When TCWM was defined as the dependent variable cheese consumption was no longer a significant predictor (beta=0.140, p=0.080). The relation between TCWM and cottage cheese was significant in the multivariate model (beta=0.203, p=0.009). The relationship between TCWM and milk-dairy protein intake remained also significant (beta=0.170, p=0.032).
When defining TCP as a dependent variable in the multiple regression model consumption of cheese turned to non-significant (beta =0.129, p=0.078) while cottage cheese consumption remained a significant predictor (beta =0.222, p=0.002). The relationship between TCP and total milk-dairy protein remained significant too (beta=0.172, p=0.018).
Analyses for males and females separately
In males cottage cheese consumption significantly correlated with TCC volume (r=0.311, p=0.032), TCWM (r=0.397, p=0.005) and TCP (r=0.398, p=0.005). In females only the relationship between cottage cheese consumption and total cerebral cortex was at the level of significance (0.233, p=0.050). After controlling for age, in males, the relationship between cottage cheese consumption and TCWM (beta=0.397, p=0.006), TCC (beta=0.310, p=0.026) and TCP (beta=0.398, p=0.005) remained significant. After controlling for age, in females, the relationship between cottage cheese consumption and TCC remained significant (beta=0.266, p=0.025).
Discussion
As far as we know this is the first study assessing the relationship between cow’s milk-dairy consumption and brain morphology. We found a number of associations between milk and dairy consumption and the investigated brain volumes. TCC, TCWM and TCP were significantly related with cottage cheese and total protein intake from milk and dairy also when controlled for age and gender in the multivariate model. Cheese consumption was significantly correlated with all three brain parameters although they turned non-significant in multivariate models.
Milk of mammalian animals is evolutionarily “designed” to promote growth of the offspring. Accordingly, breastfeeding has repeatedly been shown to confer health and cognitive benefits in later life. For example a study by Isaacs et al. (2010) reported a positive association between early breastmilk intake and brain volume as well as intelligence quotient at adolescence. Contrary to the usual belief, consumption of milk beyond breastfeeding period is associated with negative health effects too. These include iron deficiency, higher prevalence of upper respiratory tract infections, allergies, constipation and type 1 diabetes mellitus (Thorsdottir and Thorisdottir, 2011). Epidemiological and ecological data also link cow’s milk consumption to higher prevalence of certain cancers (Melnik et al., 2012) and cardiovascular disease (Laugesen and Elliott, 2003). A few studies also related cow’s milk to neurological conditions such as epilepsy, autism and sudden infant death (Thorsdottir and Thorisdottir, 2011; Lucarelli et al., 2012).
Negative effects are thought to be mediated by the bioactive substances in cow’s milk. Casein which makes up 80% of the protein in cow’s milk breaks down in the gastrointestinal tract into casomorphins such as beta casomorphin-7 (BCM-7). BCM-7 is prone to passing through the blood-brain barrier and has been suggested to exert an opioid-like effect in the central nervous system (Scientific Report of EFSA, 2009). Although this effect was described as being weak continuous exposure may represent a cumulative effect.
Cow’s milk contains IGF-1 and consumption of cow’s milk is known to elevate concentration of both IGF-1 and insulin which are regarded as key regulators of tissue growth (Melnik et al., 2012). The two latter factors together with leucine, an amino acid from milk and dairy, are known as main activating factors of the mTORC1 (mammalian/mechanistic target of rapamycin complex 1)-signaling system which promote cell growth and proliferation but inhibit apoptosis (Melnik et al., 2012).
The link between accelerated growth and cow’s milk consumption is well-established in children (Hoppe et al., 2006; Thorsdottir and Thorisdottir, 2011). Here we report that volumes of the cerebral cortex and cerebral white matter are proportionally related to milk and dairy consumption in healthy young adults. Given that the associations were strongest for milk-dairy protein, which is a summated measure of milk-dairy consumption, we suggest that the effects coming from different types of dairy are summated. Among the investigated dairy types cheese and cottage cheese were found to exhibit highest correlation measures with the brain subvolumes. This may be due to the fact that cheese and cottage cheese have the highest dry matter content and thus contains most protein with a potential biological effect. When investigating males and females separately associations were stronger for males than females, despite the smaller group size of males than females. Such a gender difference is in line with results from previous studies indicating stronger associations for males than females between milk consumption and height in children (Rogers et al., 2006) as well as breast milk intake at infancy and brain volume at adolescence (Isaacs et al., 2010).
Generally, larger brain is regarded as a positive rather than negative feature (e.g. Witelson et al., 2006). At the same time, autistic children (Hazlett et al., 2011) and patients with schizophrenia (Bassett et al., 1996) have been reported to have larger head circumference than age matched controls and brain overgrowth has been implicated in the pathology of these conditions (Hazlett et al., 2011). It is of importance too, that dietary interventions in autism rely on eliminating milk and dairy besides gluten containing foods (Elder, 2008).
In our previous study intracranial volume was inversely related with vitamin D (Plózer et al., 2014) the low serum level of which is a correlate or predictor of a wide variety of neurological and psychiatric conditions (Balion et al., 2012; Evatt et al., 2008; Holló et al., 2014). Vitamin D deficiency has been shown to promote cellular proliferation in the nervous system (Ko et al., 2004). Vitamin D is also implicated in the mTOR signaling by suppressing its activity (Lisse and Hewison, 2011). It seems that higher milk consumption and low level of vitamin D, converge in activating a common signaling pathway, and both are associated with tissue growth (Ko et al., 2004; Melnik et al., 2012) and larger brain volume as indicated by our previous (Plózer et al., 2014) and present data.
Our results indicate that dietary habits related with milk and dairy are proportionally associated with volumes of both cerebral cortex and cerebral white matter. Functional consequence of milk- and dairy-associated growth in brain parenchyma remains to be elucidated.
Acknowledgements
The authors declare that they have no conflict of interest. This work was supported by Grants SROP-4.2.1.B-10/2/KONV-2010-0002, SROP-4.2.2/A-11/1/KONV-2012-0017, PTE ÁOK-KA-2013/34039 and Hungarian Brain Research Program - Grant No. KTIA_13_NAP-A-II/9.
References
Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012 Sep 25;79(13):1397-405.
Bassett AS, Chow EW, Bury A, Ali F, Haylock CA, Smith GN, Lapointe JS, Honer WG. Increased head circumference in schizophrenia. Biol Psychiatry 1996;40:1173–5.
Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century.
Am J Clin Nutr. 2005 Feb;81(2):341-54.
Chan YH. Biostatistics 201: linear regression analysis. Singapore Med J. 2004;45(2):55–61.
Elder JH. The gluten-free, casein-free diet in autism: an overview with clinical implications. Nutr Clin Pract. 2008 Dec-2009 Jan;23(6):583-8.
Evatt ML, Delong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008 Oct;65(10):1348-52.
Gerstein HC. Cow's milk exposure and type I diabetes mellitus. A critical overview of the clinical literature. Diabetes Care. 1994 Jan;17(1):13-9.
Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008 Jul;9(7):568-78.
Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, Vachet C, Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 2011;68:467–76.
Holló A, Clemens Z, Lakatos P. Epilepsy and vitamin D. Int J Neurosci. 2014 Jun;124(6):387-93.
Hoppe C, Mølgaard C, Michaelsen KF. Cow's milk and linear growth in industrialized and developing countries. Annu Rev Nutr. 2006;26:131-73.
Isaacs EB, Fischl BR, Quinn BT, Chong WK, Gadian DG, Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr Res. 2010 Apr;67(4):357-62.
Ko P, Burkert R, McGrath J, Eyles D. Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cycle during rat brain development. Brain Res Dev Brain Res 2004;
153:61–8.
Laugesen M, Elliott R. Ischaemic heart disease, Type 1 diabetes, and cow milk A1 beta-casein. N Z Med J. 2003 Jan 24;116(1168):U295.
Lisse TS, Hewison M. Vitamin D: a new player in the world of mTOR signaling. Cell Cycle. 2011 Jun 15;10(12):1888-9.
Lucarelli S, Spalice A, D'Alfonso Y, Lastrucci G, Sodano S, Topazio L, Frediani T. Cow's milk allergy and rolandic epilepsy: a close relationship? Arch Dis Child. 2012 May;97(5):481.
Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow's milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab (Lond). 2012 Aug 14;9(1):74.
Melnik BC, Schmitz G, John S, Carrera-Bastos P, Lindeberg S, Cordain L. Metabolic effects of milk protein intake strongly depend on pre-existing metabolic and exercise status. Nutr Metab (Lond). 2013 Oct 2;10(1):60.
Plózer E, Altbäcker A, Darnai G, Perlaki G, Orsi G, Nagy SA, Schwarcz A, Kőszegi T, Woth GL, Lucza T, Kovács N, Komoly S, Clemens Z, Janszky J. Intracranial volume inversely correlates with serum 25(OH)D level in healthy young women. Nutr Neurosci. 2014 Feb 14. [Epub ahead of print]
Rogers I, Emmett P, Gunnell D, Dunger D, Holly J; ALSPAC Study Tteam. Milk as a food for growth? The insulin-like growth factors link. Public Health Nutr. 2006 May;9(3):359-68.
Scientific Report of EFSA prepared by a DATEX Working Group on the potential health impact of β-casomorphins and related peptides. EFSA Scientific Report (2009) 231, 1-107
Thorsdottir I, Thorisdottir AV. Whole cow's milk in early life. Nestle Nutr Workshop Ser Pediatr Program. 2011;67:29-40.
Witelson SF1, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain. 2006 Feb;129(Pt 2):386-98.
 Rehabilitáció csak online elérhető
Rehabilitáció csak online elérhető
 E-mail: paleomedicina@gmail.com
E-mail: paleomedicina@gmail.com


